Passivation of Stainless Steel Parts
Passivation is a critical process in industries such as aerospace, defense, electronics, and medical devices. It involves creating a protective oxide layer on the surface of stainless steel to enhance its resistance to corrosion and prolong its lifespan. Understanding the science behind passivation, exploring different methods, and examining real-world applications can help us appreciate its importance in protecting stainless steel components. In this blog, we’ll break down the key concepts, chemical reactions, benefits, challenges, and safety considerations associated with passivation. Passivation is a chemical treatment that removes free iron from the surface of stainless steel, helping to form a passive oxide layer. This layer acts as a barrier against corrosion and oxidation. If not removed, free iron can lead to rust, discoloration, and other forms of degradation. By eliminating these contaminants, passivation ensures the metal remains clean and protected, allowing the passive film to form effectively. Free Iron: Left untreated, free iron can act as a catalyst for corrosion. Removing it is essential to maintain the integrity of the stainless steel surface. Passive Film: A thin, protective oxide layer formed during passivation. It shields the metal from environmental damage and enhances durability. Surface Contamination: Dirt, oils, and grease must be removed before passivation to ensure the effectiveness of the treatment. Conversion Coating: A type of surface treatment that improves corrosion resistance. Passivation is a specific form of conversion coating designed for stainless steel. The science of passivation lies in the chemical reactions that occur when stainless steel comes into contact with a passivating solution. These reactions remove impurities and promote the formation of a stable oxide layer on the metal’s surface. Common passivation agents include citric acid and nitric acid. They react with the stainless steel, dissolving free iron and encouraging the growth of a chromium oxide layer. This layer serves as a protective shield, preventing further corrosion and improving the material’s performance in harsh environments. There are several passivation techniques, each with unique advantages depending on the application. Two common methods are citric acid passivation and nitric acid passivation. Citric acid passivation is gaining popularity due to its eco-friendly nature and lower toxicity compared to nitric acid. It is particularly suitable for sensitive industries like food processing and medical equipment. The process is safe, effective, and meets high cleanliness standards without compromising the quality of the metal surface. Nitric acid passivation is known for its strong oxidizing properties, making it ideal for removing residual iron and other contaminants quickly. It is often used in industrial settings where maximum corrosion resistance is required. However, it requires careful handling due to its higher reactivity and potential environmental impact. Oxidation plays a crucial role in forming the passive layer on stainless steel. Chromium in the alloy reacts with oxygen, creating a thin chromium oxide layer that protects the metal from corrosion. This layer prevents direct exposure to corrosive elements and enhances the overall durability of the stainless steel component. The passivation process typically includes three main steps: preparation and cleaning, applying the passivation solution, and rinsing and drying. Each step is vital to ensure the formation of a strong and uniform passive layer. Before passivation, the stainless steel parts must be thoroughly cleaned to remove oils, dirt, and other contaminants. Proper cleaning ensures that the passivation solution can work effectively on the surface. Stainless steel parts are immersed in a passivation solution, either citric or nitric acid, depending on the desired outcome. The immersion time varies based on the type of stainless steel and the method used. After passivation, the parts are rinsed to remove any remaining chemicals. Drying is essential to prevent water spots and ensure the long-term stability of the passive layer. While passivation offers many benefits, it also presents some challenges. For example, maintaining the integrity of the passive layer may require periodic treatments, especially in highly corrosive environments. Additionally, improper passivation can lead to issues like staining, discoloration, or even accelerated corrosion. To avoid problems during passivation, it's important to follow proper procedures, use the right chemicals, and ensure thorough cleaning before and after the process. Working with experienced professionals can also help achieve better results and reduce the risk of failure. Safety is a top priority during passivation due to the use of strong acids. Always wear appropriate personal protective equipment (PPE), such as gloves and goggles. Work in well-ventilated areas and follow manufacturer guidelines for solution concentrations. After passivation, rinse parts thoroughly and dispose of waste according to local regulations to ensure a safe working environment. Passivation is a chemical process that removes free iron from stainless steel surfaces, creating a protective oxide layer that improves corrosion resistance and enhances the metal's longevity. Yes, stainless steel benefits from passivation, especially after fabrication or machining. It helps maintain the material’s integrity and performance in various environments. Common passivation chemicals include citric acid, nitric acid, and sodium dichromate. Each has its own advantages and is chosen based on the application and industry requirements. Carbon steel cannot be passivated in the same way as stainless steel. Instead, it requires protective coatings like oil, paint, or other inhibitors to prevent corrosion. These coatings are more prone to wear and require regular maintenance. Factors include the chromium content of the steel, correct immersion time, surface condition, temperature, and solution concentration. Proper execution of each step is essential for optimal results. Storage Systems, Adjustable with Your Need,Different Colours Order,Different Colors Can Be Ordered Changzhou Fuku Precision Machinery Co., Ltd. , https://www.fukuindustrial.comPassivation of Stainless Steel Parts
Understanding Passivation
Key Terms in Passivation
The Science Behind Passivation
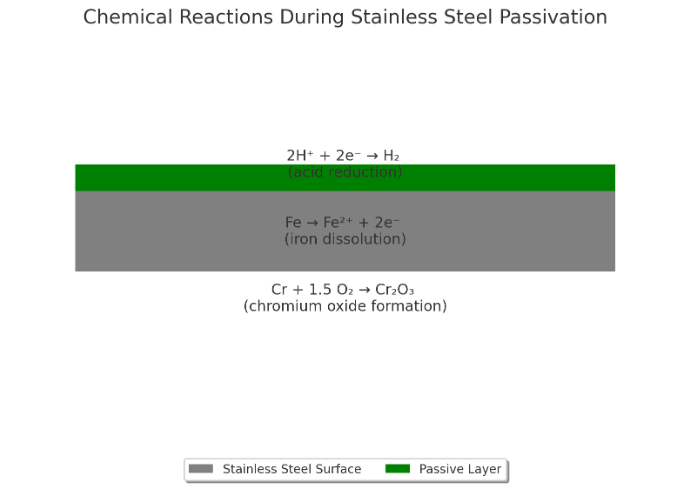
Types and Techniques of Passivation
Citric Acid Passivation
Nitric Acid Passivation
The Role of Oxidation in the Passivation Layer
The Procedure of Passivating Stainless Steel
1) Preparation and Cleaning
2) Applying Passivation Chemical
3) Rinsing and Drying

The Challenges of Passivation
Potential Issues and How to Avoid Them
Safety Measures During Passivation Process
Key Equipment in Stainless Steel Passivation
 In industrial passivation, specialized equipment such as acid-resistant tanks, temperature-controlled baths, spray rinse stations, and drying ovens are essential. Automated systems like conveyors and robotic arms help streamline the process, while pH meters and solution analyzers ensure consistent quality. Protective gear is also necessary to safeguard operators during the procedure.
In industrial passivation, specialized equipment such as acid-resistant tanks, temperature-controlled baths, spray rinse stations, and drying ovens are essential. Automated systems like conveyors and robotic arms help streamline the process, while pH meters and solution analyzers ensure consistent quality. Protective gear is also necessary to safeguard operators during the procedure.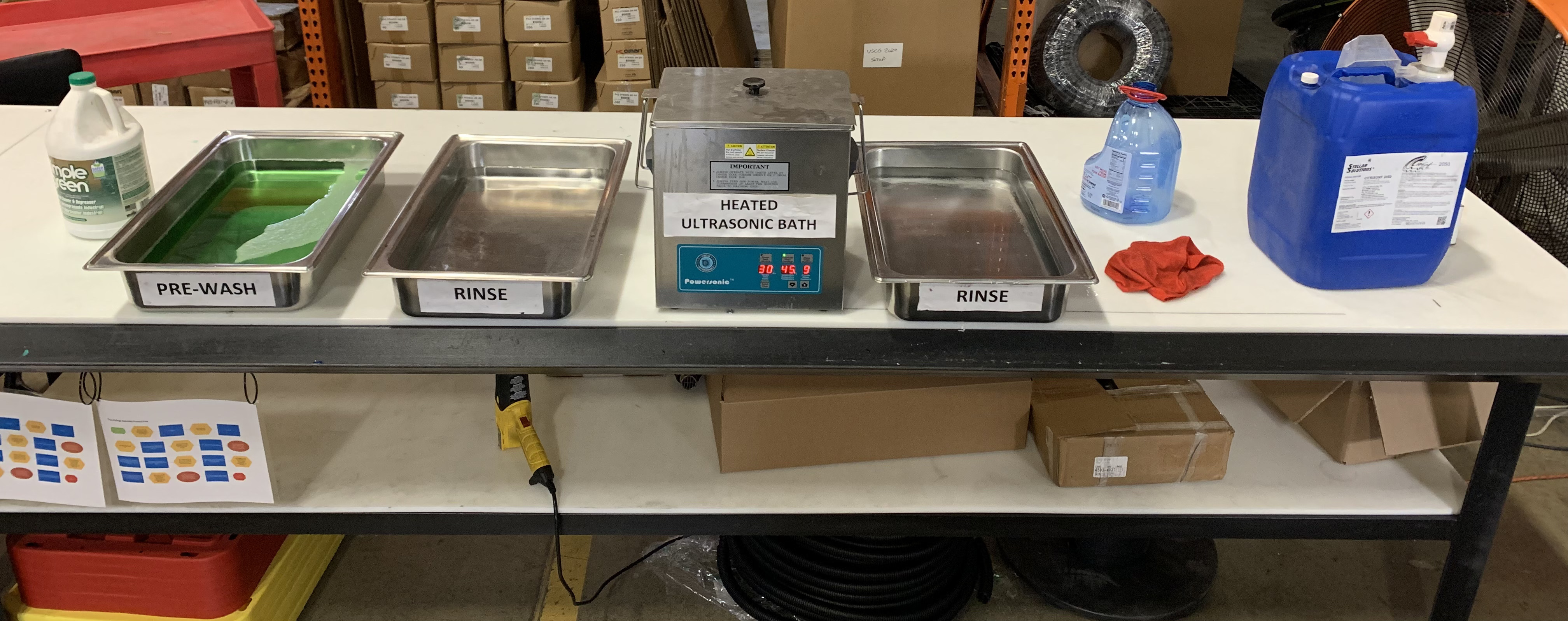
Frequently Asked Questions
What is passivation?
Does stainless steel need passivation?
What chemicals are used in passivation?
Can I passivate carbon steel?
What Factors Influence the Success of the Passivation Process?