Passivation of Stainless Steel Parts
Passivation plays a vital role in the manufacturing and maintenance of stainless steel components across various industries, including aerospace, defense, electronics, and medical. This process enhances the material's corrosion resistance by forming a protective oxide layer on its surface. Understanding the science behind passivation, the different methods used, and the benefits it provides is essential for ensuring the longevity and performance of stainless steel parts. In this article, we'll explore the fundamentals of passivation, its chemical mechanisms, and practical applications, while also addressing challenges and safety considerations. Passivation is a chemical treatment designed to improve the corrosion resistance of stainless steel by removing free iron from the surface and promoting the formation of a passive oxide layer. Free iron can act as a catalyst for rust and oxidation, especially when exposed to moisture or harsh environments. By eliminating these contaminants, passivation creates a clean and stable surface that significantly reduces the risk of corrosion and discoloration over time. Free Iron: Residual iron particles on the surface can lead to corrosion if not removed. Passivation helps eliminate these impurities, ensuring a more durable finish. Passive Film: A thin, protective oxide layer formed during passivation, typically composed of chromium oxide. This film acts as a barrier against corrosive elements. Surface Contamination: Dirt, oil, grease, or other foreign substances must be removed before passivation to ensure effective treatment and long-term performance. Conversion Coating: A broader term referring to chemical treatments that enhance surface properties. Passivation is a specific type of conversion coating tailored for stainless steel. The core principle of passivation lies in the chemical reactions that occur between the stainless steel surface and the passivating solution. When immersed in a solution such as citric acid or nitric acid, the metal reacts to form a dense, protective oxide layer. This layer prevents further interaction with environmental elements like oxygen and moisture, thereby enhancing the material’s durability and aesthetic appearance. The effectiveness of passivation depends on factors such as the type of acid used, immersion time, temperature, and the composition of the stainless steel alloy. Understanding these variables allows for optimized results and consistent quality in industrial applications. There are several passivation techniques available, each with unique advantages depending on the application and industry requirements. The two most commonly used methods are citric acid passivation and nitric acid passivation, both of which offer effective ways to protect stainless steel surfaces. Citric acid passivation has gained popularity due to its eco-friendly nature and lower toxicity compared to traditional methods. It is particularly favored in medical and food processing industries where cleanliness and safety are critical. This method effectively removes surface impurities without the need for harsh chemicals, making it a safer and more sustainable option for many applications. Nitric acid passivation is known for its strong oxidizing properties, which allow it to remove residual iron and other contaminants quickly. This technique is often used in high-performance environments where maximum corrosion resistance is required. However, it requires careful handling due to its stronger chemical reactivity and potential hazards. Oxidation is a key factor in the formation of the passive layer on stainless steel. Chromium, a primary component of stainless steel, reacts with oxygen in the passivation solution to form a thin chromium oxide layer. This layer acts as a shield, preventing further corrosion and improving the material’s overall performance. The passivation process involves three main steps: preparation and cleaning, application of passivation chemicals, and rinsing and drying. Each step is crucial for achieving an effective and long-lasting protective layer on the stainless steel surface. Before passivation, the stainless steel surface must be thoroughly cleaned to remove oils, dirt, and other contaminants. Proper cleaning ensures that the passivation solution can work effectively and that the resulting oxide layer is uniform and durable. Stainless steel parts are immersed in a passivation solution, such as citric acid or nitric acid, for a specified duration. The exact time and concentration depend on the type of stainless steel and the desired outcome. Following ASTM standards is recommended to ensure consistency and quality. After the passivation treatment, the parts must be rinsed thoroughly to remove any remaining chemicals. Proper drying is essential to prevent water spots and maintain the integrity of the protective oxide layer. While passivation offers significant benefits, it also comes with certain challenges. These include the need for regular maintenance, the risk of improper application leading to reduced effectiveness, and the importance of following proper safety protocols. Understanding these challenges helps in implementing more reliable and efficient passivation processes. Safety is a top priority when performing passivation due to the use of strong acids and chemicals. Operators should wear appropriate personal protective equipment (PPE), such as gloves, goggles, and lab coats. Work areas should be well-ventilated to minimize exposure to fumes. Additionally, all chemicals must be handled and disposed of according to local regulations to ensure a safe working environment. Passivation is a chemical process that removes free iron from the surface of stainless steel, creating a protective oxide layer that enhances corrosion resistance and prolongs the life of the material. Yes, stainless steel benefits from passivation, especially after fabrication or machining. It improves the material’s durability and resistance to corrosion, making it suitable for demanding applications. Common chemicals used in passivation include citric acid, nitric acid, and sodium dichromate. Each has its own advantages and is chosen based on the specific needs of the application. Carbon steel can be treated with protective coatings or inhibitors, but it does not form a natural passive layer like stainless steel. These treatments require more frequent maintenance and are less self-healing than the oxide layer on stainless steel. Factors include the chromium content of the steel, correct immersion time, surface grain structure, temperature, and solution concentration. Each of these plays a role in determining the effectiveness of the passivation treatment. Frame,Size and Figure Customized,Welding is Close Seam,Brushed Nickel Surface Treatment Changzhou Fuku Precision Machinery Co., Ltd. , https://www.fukuindustrial.comPassivation of Stainless Steel Parts
Understanding Passivation
Key Terms in Passivation
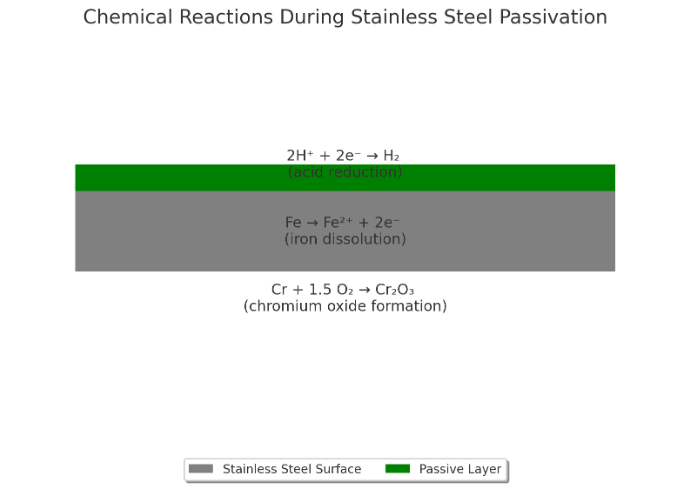
The Science Behind Passivation
Types and Techniques of Passivation
Citric Acid Passivation
Nitric Acid Passivation
The Role of Oxidation in the Passivation Layer
The Procedure of Passivating Stainless Steel
1) Preparation and Cleaning
2) Applying Passivation Chemicals
3) Rinsing and Drying

The Challenges of Passivation
Safety Measures During Passivation Process
Key Equipment in Stainless Steel Passivation
 In industrial settings, specialized equipment such as acid-resistant tanks, spray rinse stations, and drying ovens are essential for efficient passivation. Automated systems like conveyors and robotic arms help streamline the process, while tools like pH meters and solution analyzers ensure accurate monitoring of the treatment.
In industrial settings, specialized equipment such as acid-resistant tanks, spray rinse stations, and drying ovens are essential for efficient passivation. Automated systems like conveyors and robotic arms help streamline the process, while tools like pH meters and solution analyzers ensure accurate monitoring of the treatment.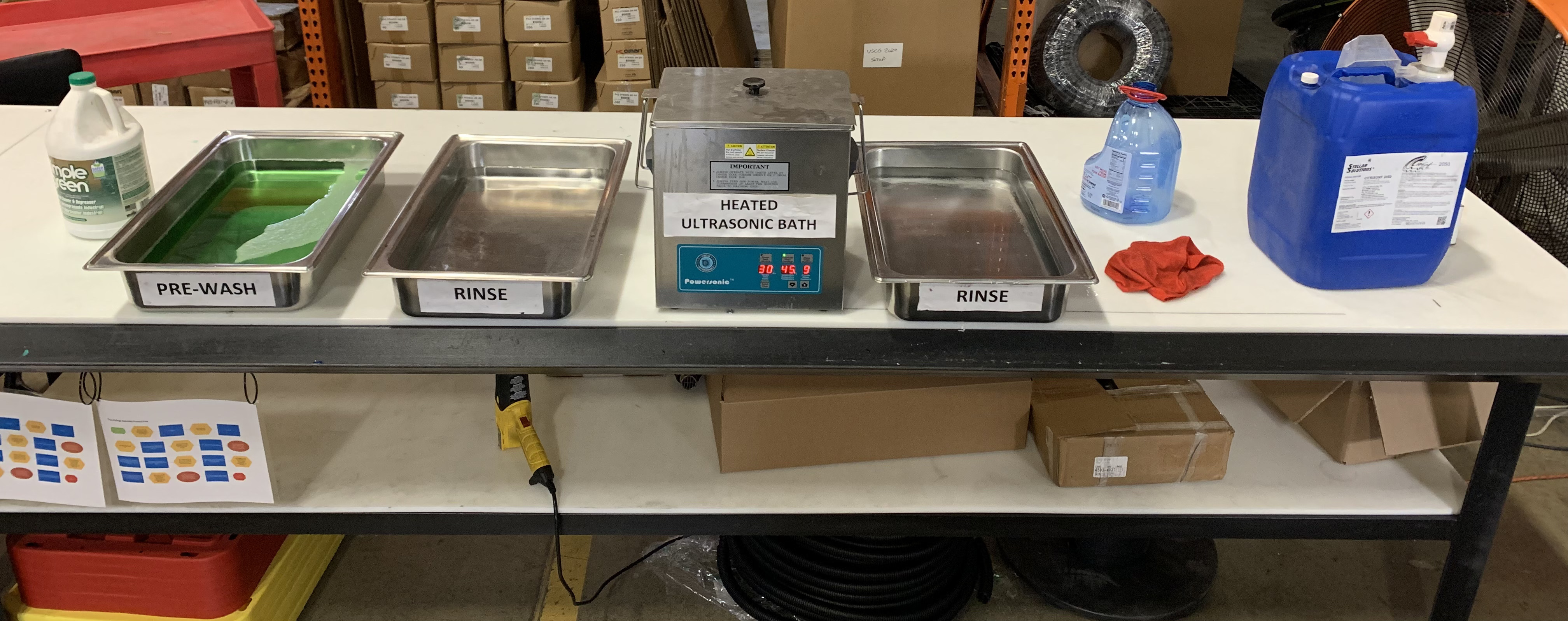
Frequently Asked Questions
What is passivation?
Does stainless steel need passivation?
What chemicals are used in passivation?
Can I passivate carbon steel?
What Factors Influence the Success of the Passivation Process?